Abstract
Background. Early mortality remains a challenging therapeutic facet of the initial induction phase of intensive chemotherapy in patients with acute myeloid leukemia (AML). The impact of standard molecular evaluation and risk category of the European LeukemiaNet (ELN) 2017 classification model on early mortality have not been rigorously evaluated thus far.
Aim. Evaluate the impact of standard clinical, laboratory, cytogenetic, and molecular parameters on the likelihood of early death following induction chemotherapy in a cohort of uniformly treated AML patients receiving standard intensive induction chemotherapy.
Methods. We reviewed the medical records of 320 consecutive adult patients with newly diagnosed AML treated with intensive induction chemotherapy in our center from 2007 to 2021. The Kaplan-Meier method was used to determine overall survival and the log-rank test was used to compare differences between groups. All tests were two-sided and a p-value < 0.05 was considered statistically significant.
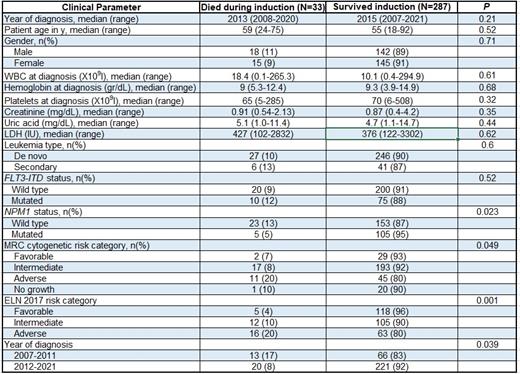
Results. From October 2007 to January 2021, 320 adult patients with AML were treated with standard anthracycline and cytarabine-based intensive induction chemotherapy . Median age was 56 years (range, 18 to 92); 33 patients (10%) died during induction. As outlined in table 1, patient age, white blood cell count, hemoglobin level, platelet level, creatinine, uric acid, lactate dehydrogenase serum levels, FLT3-ITD and CEBPA mutational status did not significantly impact on early mortality. NPM1 mut patients had a lower likelihood of early death compared to NPM1 wt (5% versus 13%; p=0.023) whereas patients with high-risk cytogenetic studies experienced higher rates of induction mortality compared with intermediate and favorable risk patients (20% versus 8% and 7%, respectively; p=0.049). Adverse risk ELN 2017 were significantly more likely to die during induction compared with intermediate and favorable risk patients (20% versus 10% and 4%, respectively; p=0.001). Patients treated in 2007-2011 experienced a significantly higher rate of induction death compared with patients in 2012-2021 (17% versus 8%; p=0.039). Multivariate analysis confirmed adverse ELN 2017 [odds ratio (OR), 6.7; 95% confidence interval (CI), 1.77-25.3; p=0.005) and treatment timeframe (OR, 0.36; 95% CI, 0.15-0.87; p=0.023) as pivotal predictors of early mortality.
Conclusion. Our data underscore the tight association of the ELN 2017 risk model with early induction mortality in AML patients receiving intensive induction chemotherapy. With the introduction of novel agents as well as innovative regimens combining these agents, it is becoming clear that better prognostic tools are needed at the provider level to navigate the rapidly changing therapeutic landscape seen in current management of AML patients. Our findings further underpin the importance of integrated prognostication models and their profound implications on the immediate peri-induction setting in AML patients.
Avigdor: Gilead: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Research Funding; Janssen: Research Funding; Takeda: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal